Electro Polishing
Electrolytic polishing effective for high hygiene control and your product composition stability

GOLD EP Surface-treated industrial cleaning shower balls
Electrolytic polishing (Electro polishing, electropolishing, EP) uses an electrolytic reaction to melt metal surfaces such as stainless steel, titanium, and aluminum. This process is used to melt ultra-fine irregularities and turn the objects into smoothed and mirrored metal surfaces. A homogeneous, chrome-rich passivation film with excellent corrosion resistance is formed on the electropolished metal surface.
Remove dirt on the metal surface and make it hard to get dirty
Even if stainless steel is buffed, there is still fine burrs, scratches, particles, etc. remaining on the surface. In the case of pipes and tanks used in food and chemicals factories, it may lead to quality and safety problems such as the growth of germs due to stagnant substances, the effect on product composition, and corrosion of metal surfaces.
(1) Removes impurities such as abrasives, metal powder, and oils and fats (2) Form a smooth surface that is hard to get dirty and easy to clean.
Cover the metal surface with a homogeneous film and Create a ground where the film can be easily regenerated even if it is damaged
Stainless steel is an alloy with excellent corrosion resistance against rust, which causes various problems. This characteristic is achieved thanks to the passivation film (chromium oxide layer) covering on the metal surface. (3) It is necessary to form a perfect film so that there is no gap for rust, and (4) make it a base that can be easily repaired automatically so that it can withstand long-term use.
For electrolytic polishing, stainless steel, which is easy to process and does not rust easily at the surface, is further strengthened with (1) to (4). Those steps keep the inside of stainless steel pipes and tanks clean at all times.
Content (with links)
1.Passivation film of stainless steel
2.Advantages and features of electrolytic polishing
3.Electrolytic polishing equipment
4.Principles of electrolytic polishing
5.State near the surface after electrolytic polishing
6.More advanced electrolytic polishing
7.Electrolytic polishing for materials other than stainless steel
8.Necessity to consider requirements for processing steps before and after electrolytic polishing
1.Passivation film of stainless steel
Stainless steel is an iron alloy that is very useful as it does not rust easily. In addition to stainless steel (iron), titanium and aluminum have the property of being easily corroded chemically, but they also have the property of repelling rust due to the oxide film (passivation film) formed on the surface.
Even if the passivation film of stainless steel is damaged, chromium (Cr) in the stainless steel combines with the outside air and moisture, resulting in the film being automatically repaired immediately, so stainless steel is used in various environments.
Electrolytic polishing (Electro polishing, EP), as the name implies, also smoothens the metal surface by electrolytic dissolution, and the more important point is making a passivation film in perfect condition for protection and repair.
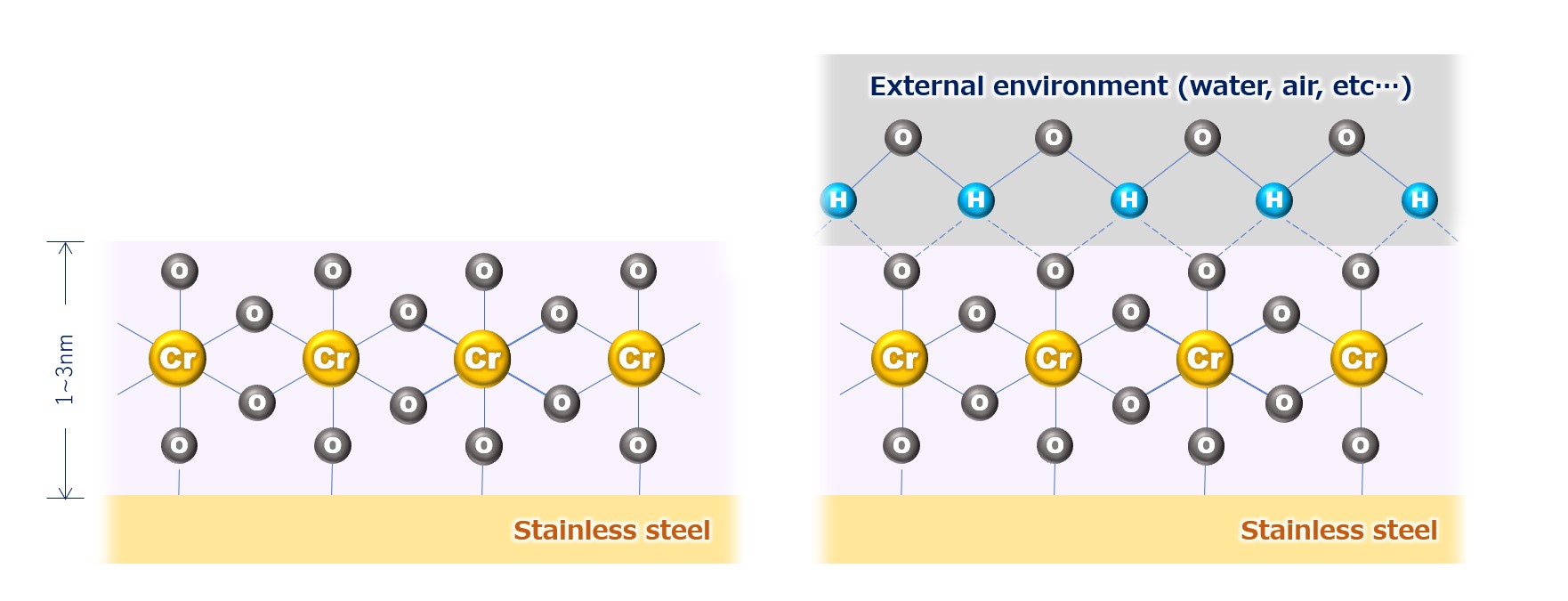
Fig.1: chromium oxide passivation film of stainless steel
* The passivation film of stainless steel is chromium oxide (left side of the figure), but in the actual environment (atmosphere, etc.), it exists in the state of hydrated oxyhydroxide combined with water and oxygen (right side of the figure).
Even if the object is stainless steel material for sanitary use, the surface condition of the metal due to polishing and processing is not as perfect as the molecular model shown in Fig. 1 due to scratches, stains, and alteration. It is common knowledge that “stainless steel does not rust easily”. However, in order to fully exploit its capabilities, the surface condition of stainless steel must be physically and molecularly prepared so that it can respond quickly and properly to corrosion. Electrolytic polishing (Electro polishing, EP) is an electrolytic process that provides physical smoothing and chemical homogenization to turn the surface of stainless steel into the best possible condition.
2.Advantages and features of Electrolytic polishing (EP)
(1) Features of Electrolytic polishing (EP)
-
The treated surface is smoothed and mirrored
-
A dense passivation film is formed
-
Covered with a layer rich in chromium(chromium-rich layer) with excellent corrosion resistance
(2) Advantages of Electrolytic polishing (EP)
-
Removing dirt
The processed alteration layer is removed and the effect on corrosion resistance is reduced. -
Hard to get dirty
Fine powder and dirt are less likely to adhere and stay. The effect of them on the product is unlikely to appear.
Cleaning becomes easier and the load of clean maintenance is reduced. -
Preventing rust
The rust preventive effect is improved, and the effect of corrosion on the product and leakage accidents are less likely to occur.
Improved resistance to chemicals and food materials, making it easier to maintain quality.
(3) Why is Electrolytic polishing (EP) necessary? Hidden problems in the work-affected layer
Problems of work-affected layer
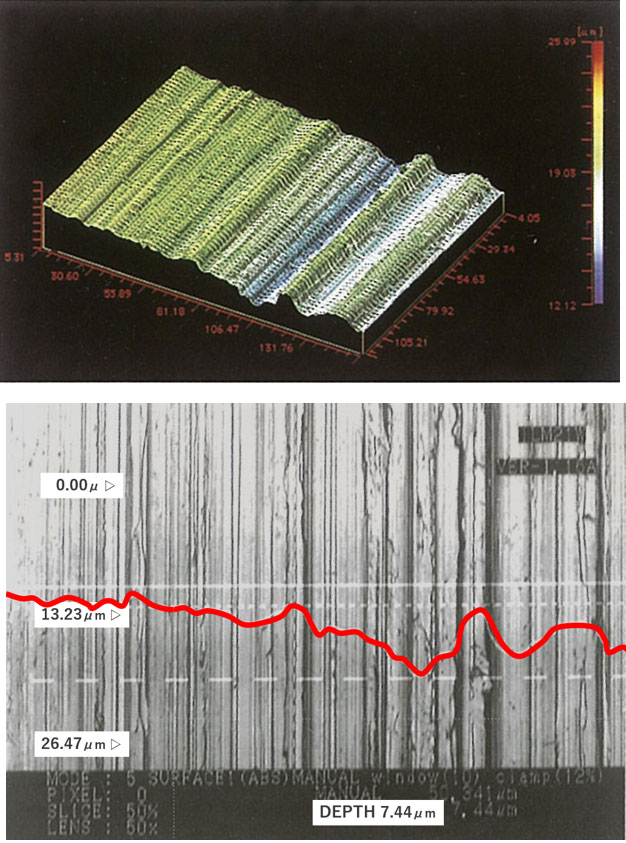
The work-affected layer is the surface layer of metal whose metal material has changed mechanically and thermally due to processing such as mechanical polishing, and is a place where energy is high, meaning chemically unstable, and is easily changed. In reality, it is in a complicated state due to streaks and scratches caused by polishing, and dirt due to abrasives and organic substances. Processed stainless steel pipes and the like are wrapped in such an unstable layer.
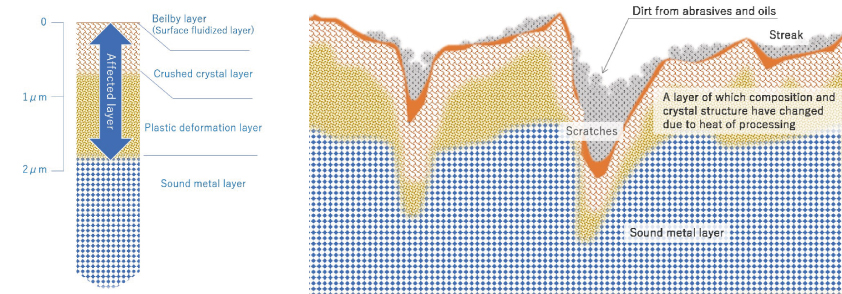
[Photo 2-3-1] Potential fragility of work-altered layer and metal surface
※ Fig. 2 is an image for explaining the metal surface. It is an exaggerated imaginary view, not a diagram of an actual cross-sectional photograph.
Corrosion resistance of stainless steel can be improved by forming a passivation film on the metal surface. However, when the surface condition is microscopically rough, a clean and homogeneous passivation film is hard to be achieved even when an oxide film is formed by passivation.
Even if it is visually close to a mirror surface, there is a possibility that the cause of troubles such as future corrosion and elution of impurities may be inherent, and it is undeniable that it may lead to quality problems in a severe production environment.
Electrolytic polishing (Electro polishing, EP) is soil improvement & leveling work
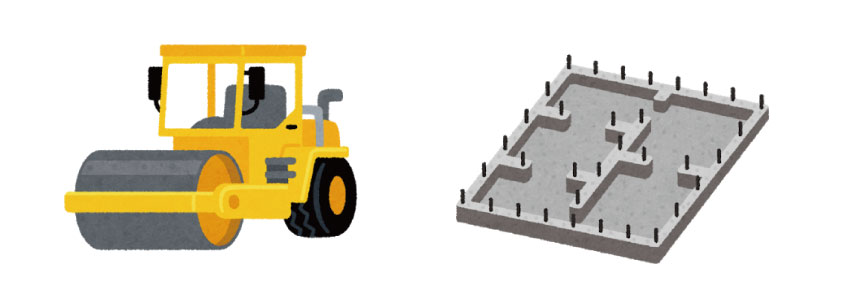
De-polishing treatment improves this unstable work-affected layer and maximizes the inherent properties of stainless steel. It’s like redevelopment of the site.
[Removing of scrap etc.]
Removes adhering and accumulated contaminants
[Breaking fragile rocks and filling wetlands]
Melts parts that are structurally unstable in metal composition
[Leveling flat with construction equipment]
Flattening unevenness on the metal surface
[Putting crushed stone and pouring concrete]
A homogeneous immobile film is formed on the prepared metal surface.
This creates a stable land that does not allow sediment to flow even in rainwater, does not crack in the sun, does not cause depressions or uplifts, is easy to clean, and is easy to pass through.
Also, by leveling, the surveyed area of the land is almost the same as the total surface area of the actual surface including hills and dents, and is minimized. Similarly on metal surfaces, minimizing the contact area with the environment and fluids also minimizes the possibility of potential troubles.
Beilby layer:
Polishing a metal creates a fluid layer on its surface that is approximately 1-10 nm thick. It fills the irregularities on the metal surface and creates an apparently smooth surface. The layer looks like a polished surface of the metal, but exhibits non-metal properties and is chemically unstable.
Plastic deformation:
Deformation in which the deformation due to the external force remains even if the applied external force is removed. The deformation that returns to the original is called elastic deformation, but when it exceeds the limit, plastic deformation occurs and the shape cannot be restored.
Passivation:
Stainless steel forms an oxide film even in the atmosphere, but it is easily broken because it is thin and inhomogeneous. Therefore, it is a treatment that forcibly oxidizes the surface by immersing it in a liquid such as nitric acid to enhance corrosion resistance. It also has the effect of removing pollutants.
3.Electrolytic polishing equipment
The material is put into an electrolytic polishing solution, and a metal material is used as an anode to conduct direct current.
The actual electrolytic polishing is not as simple as the model below, and the shape and arrangement of the electrodes are adjusted according to the shape of the material and the part to be electropolished. We receive many requests for electro polishing of complicated shapes such as prefabricated pipes and custom-made containers and the inner surface, and we use various know-how to perform electrolysis control.
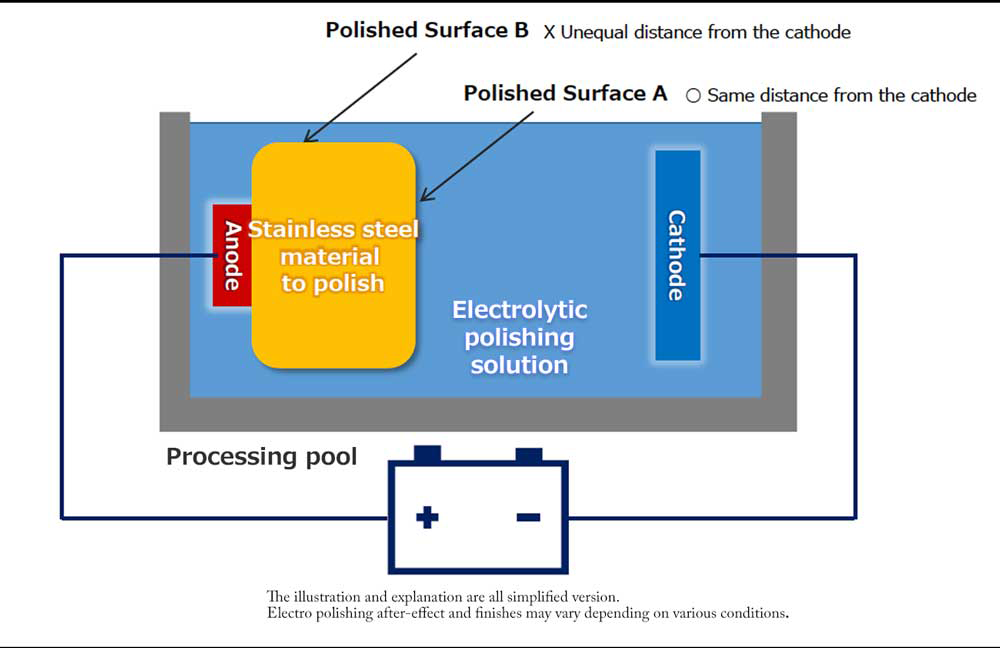
【Fig 3-1】Configuration of electrolytic polishing equipment
*Electrolytic polishing is a microscopic chemical reaction process that does not remove large irregularities.
*It is necessary to pre-treat the stainless steel material by buffing etc. in advance.

【Picture 3-2】The electrolytic polishing equipment of VINA ASTEC, subsidiary in Vietnam
4.Principle of Electrolytic polishing (Electro polishing, EP)
Below is the explanation of the principle of electrolytic polishing (EP) using sanitary material (stainless steel) as an example.
(1) Electrolytic polishing of sanitary materials
When the image of the surface of the sanitary material is enlarged, we can see many polishing marks and scratches that are less than 1/100 mm. There is also contamination by fine particles. I will explain the process of smoothing these irregularities by electrolytic polishing.
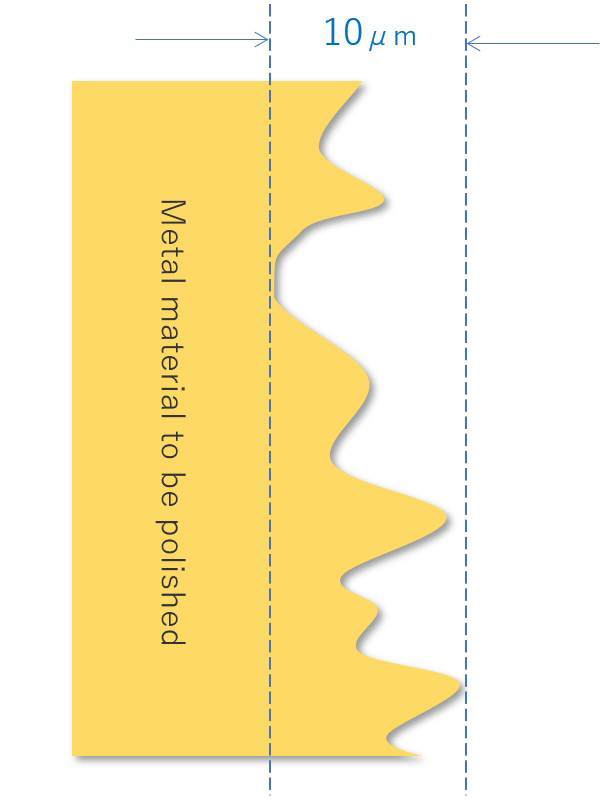
【Figure 4-1】Sectional diagram of the metal material to be polished
【Figure 4-2】Surface condition of metal material
*SI symbol unit:
1μm length equaling 1×10−6 meter 1μm=0.001mm=1,000nm
(2) Immersion in electrolytic polishing solution liquid
Connect the sanitary material to be electropolished to the anode (+) of the DC power supply and immerse it in the electrolytic polishing solution. Place the cathodes so that the entire electropolished surface (or each surface to be polished) of the metal material is approximately equal.
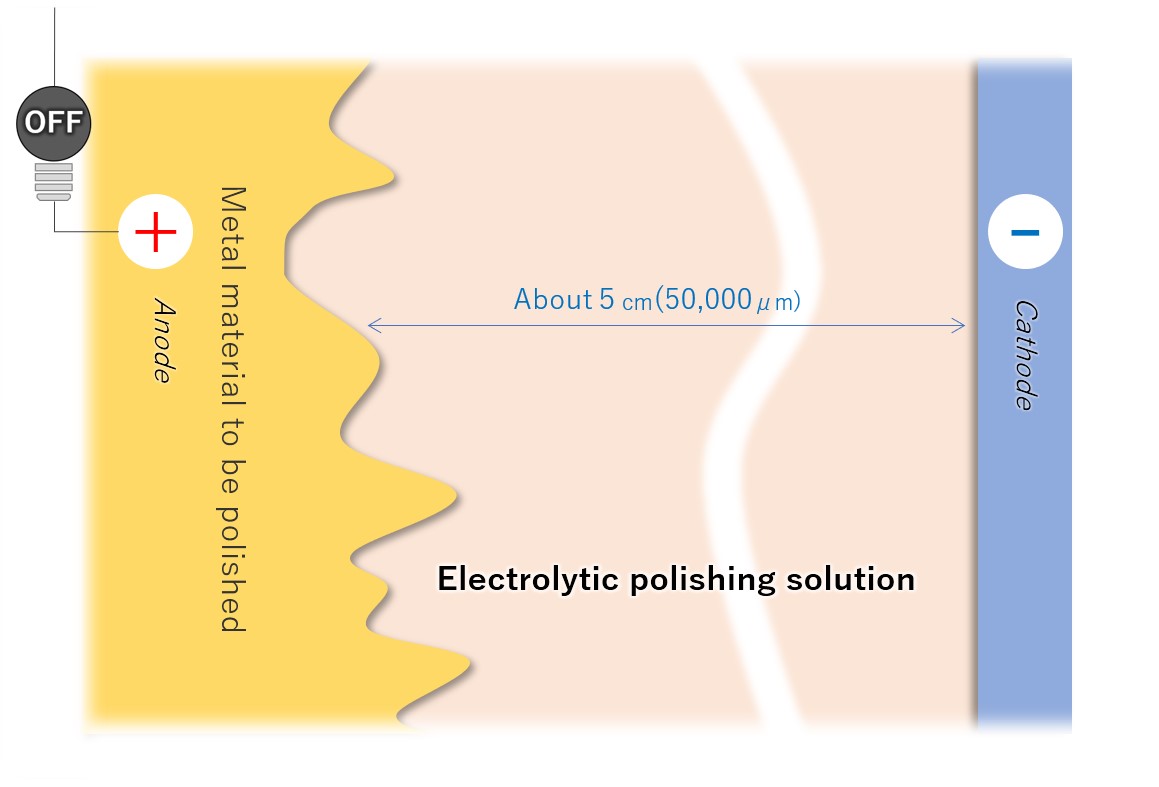
【Figure 4-3】Immerse the material in the electrolytic polishing solution
(3)Formation of a viscous initial oxide layer
When energized, the metal material on the anode side reacts with the electrolytic solution to form a viscous initial oxide layer. The distance (interface) between this layer and the electrolytic polishing solution liquid is almost flat. If the cathode is placed properly, the interface and the electrode will face each other at almost equal distances.
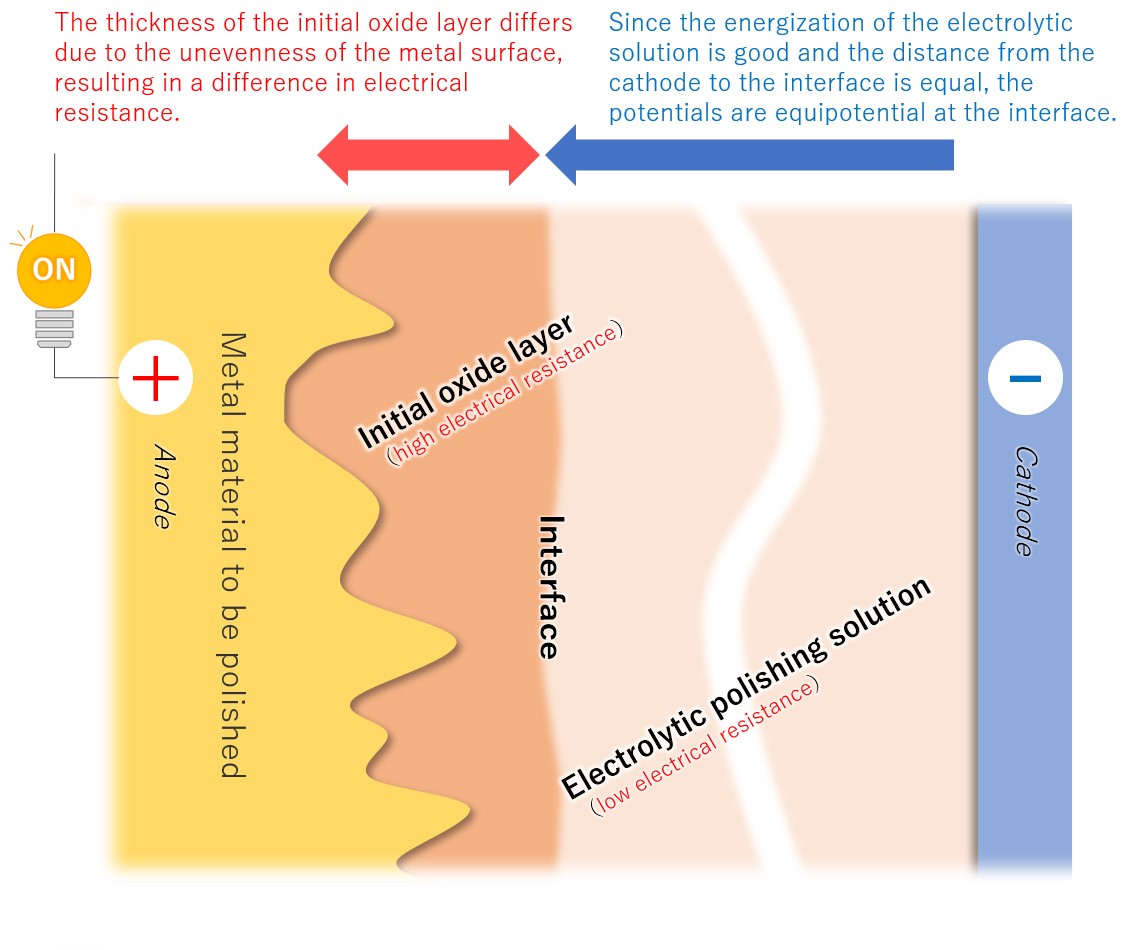
【Figure 4-4】Formation of viscous initial participating layer
Compared to an electrolytic solution with good energization, the initial oxide layer has an electric resistance of about an order of magnitude, making it difficult for electricity to pass through. Therefore, the potential is almost equipotential from the cathode to the interface, but from there to the metal, the thickness of the initial oxide layer becomes electrical resistance and a large change occurs in the current flow.
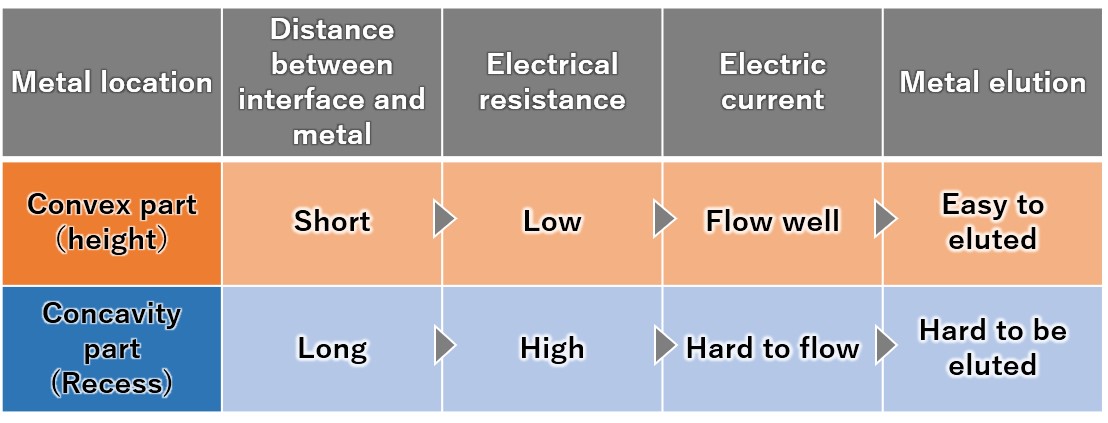
(4) Priority elution from the convex part of the metal
Since the metal convex part protruding toward the interface has low electrical resistance, a large current flows and dissolution proceeds. Since the metal recess has a distance to the interface and current does not easily flow, it is difficult for melting to occur.
Therefore, the convex parts of the metal are selectively and preferentially melted, and the metal surface gradually becomes flat.
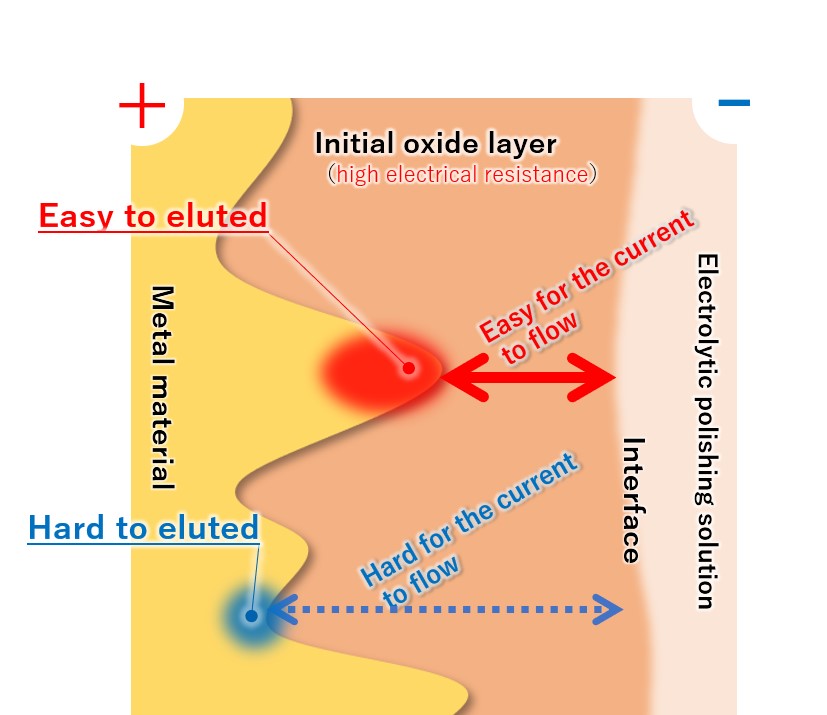
【Figure 4-5】Selective metal elution
【Figure 4-6】The reason why the convex parts of the metal surface are selectively eluted
(5) Termination of electrolytic polishing action and formation of passivation film
As the melting progresses and the metal surface becomes almost flat, the distance between the interface and the metal becomes equal. Then, it becomes difficult for the electric current to flow uniformly anywhere in the metal, and the effect of electrolytic polishing gradually diminishes.
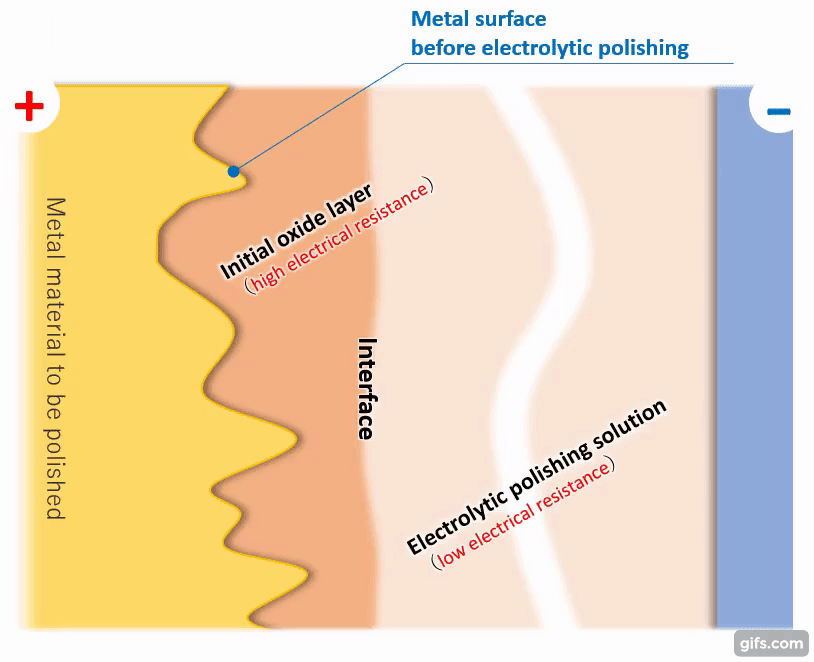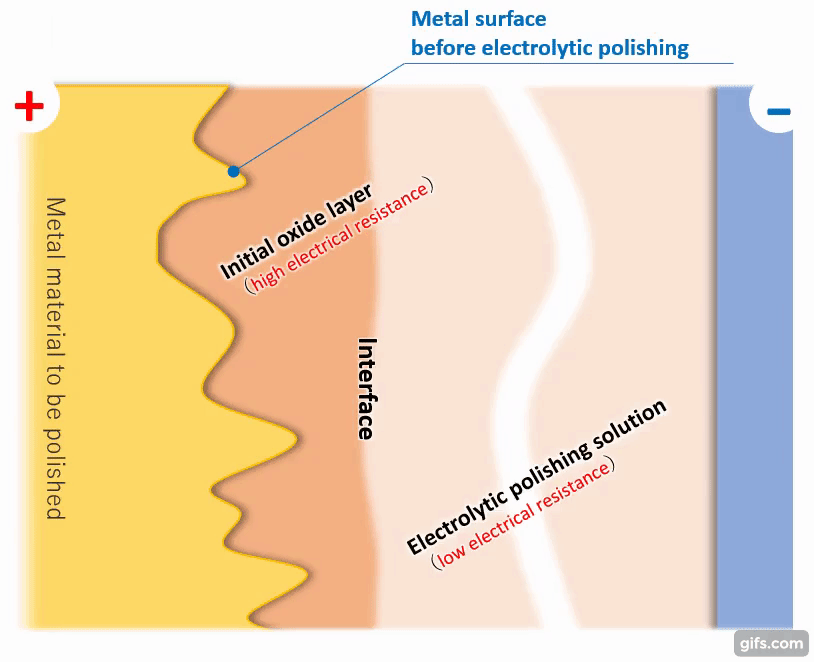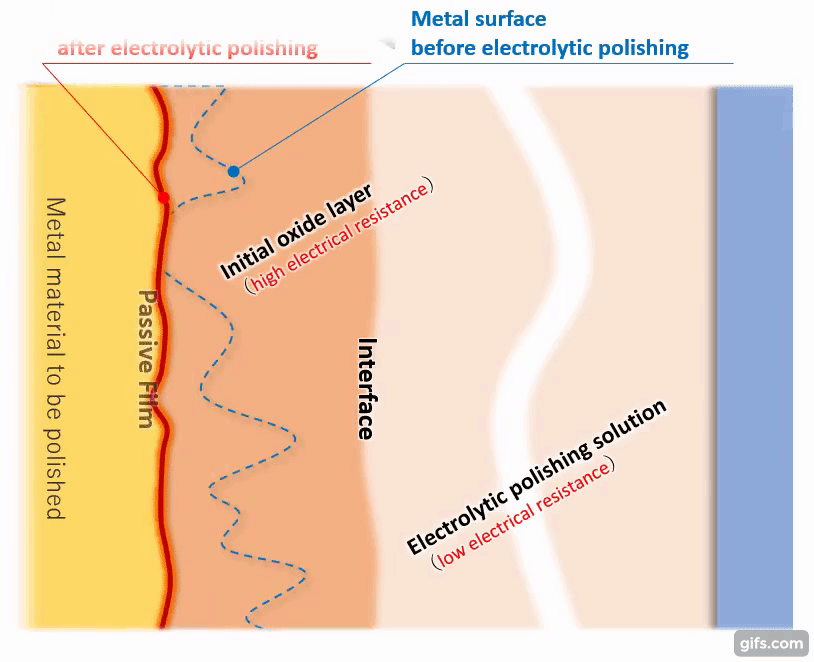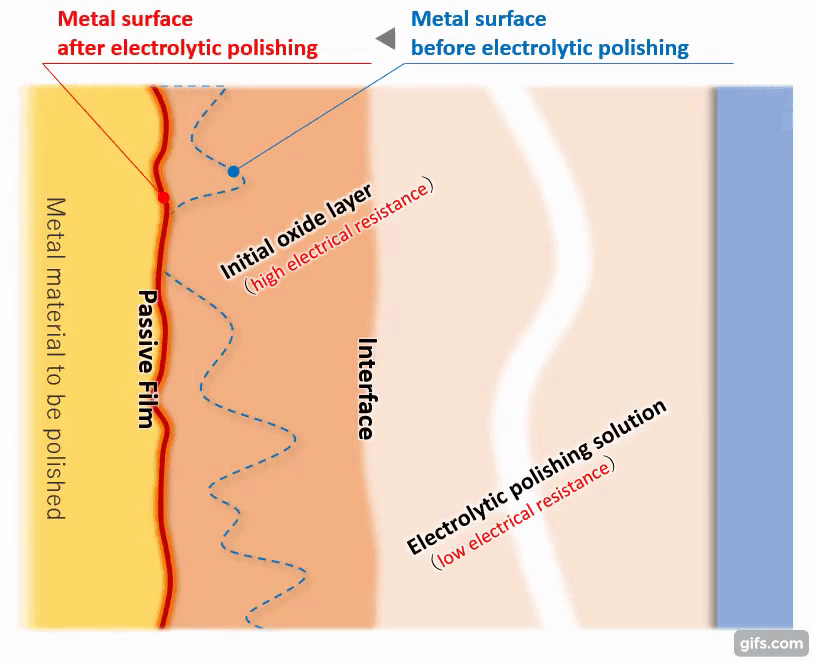
【Figure 4-7】Condition of metal after electrolytic polishing
An extremely homogeneous and strong passivation film (chromium oxide) is formed on the flattened metal surface to cover the metal piece. Even if there is an unformed part of the film, the current flows preferentially there, which promotes the film formation and as a result, a homogeneous passivation film is formed as a whole.
*The thickness of the passivation film by normal electrolytic polishing is approximately 1 to 3 nm (nanometers).
5.State near the surface after electrolytic polishing
(1) Homogeneous passivation film
When buffing a stainless steel sanitary material, the passivation film that was originally formed is scraped off, so it is usually soaked in nitric acid or the like to forcibly form a passivation film (Nitric acid immersion method / passivation treatment). However, it is difficult to form a homogeneous passivation film on a “micro-coarse” metal surface with this method.
As mentioned above, electrolytic polishing (EP) removes dirt and forms a homogeneous passivation film with no unformed parts on a smoothed good metal surface.
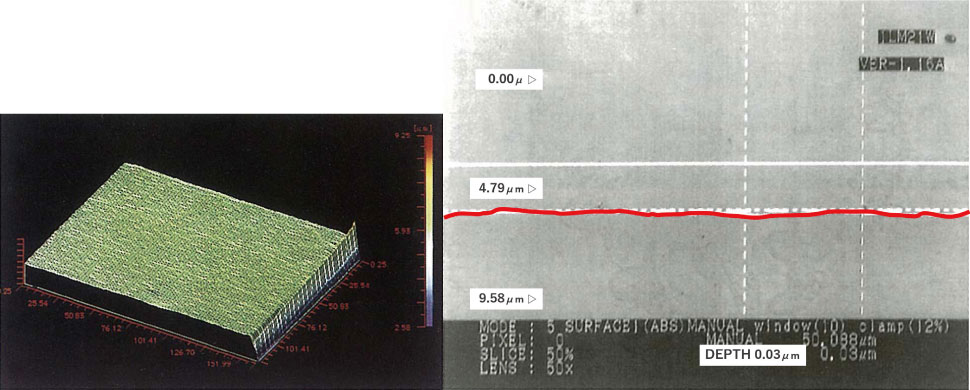
【Figure 5-1】Metal material surface condition – after electrolytic polishing
(2) Strengthen self-repairing ability! Chrome-rich surface
As shown in Fig. 6 below, the metal surface layer after electrolytic polishing has a chromium (Cr) concentration of 60% (30% iron), which is usually about 18% in SUS316 stainless steel, at the surface layer 1 to 3 nm. You can see that it is in a state of higher corrosion resistance which is rich in chrome.
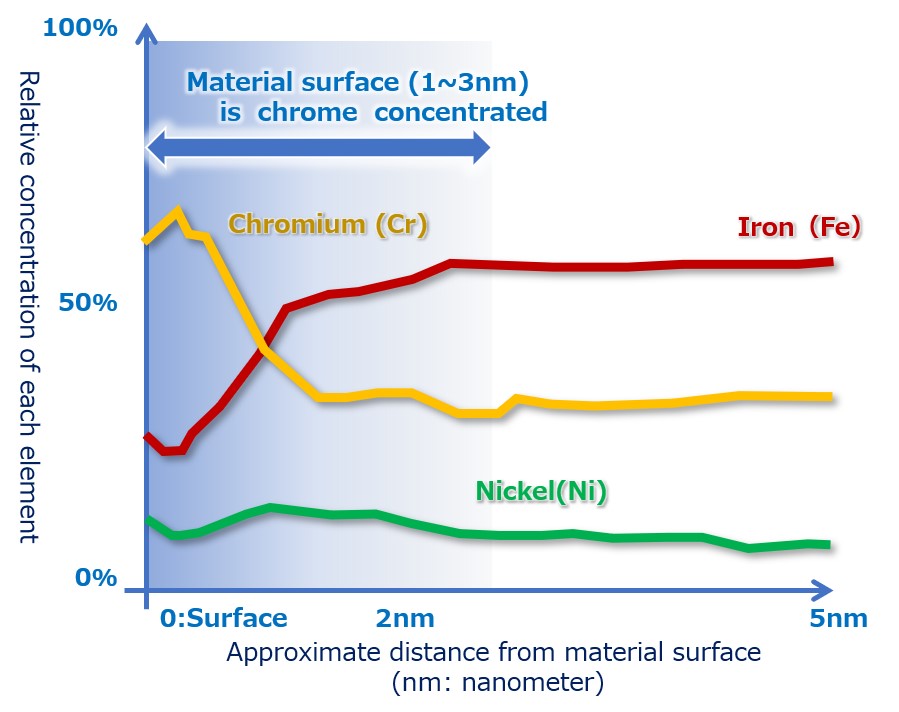
【Figure 5-2】Condition of stainless steel surface after electrolytic polishing its chrome concentrated
6.More advanced electrolytic polishing – GOLD EP / PLATINUM EP
We have technology and know-how to perform GOLD EP ( or G-EP) and PLATINUM EP (or P-EP) that form a stronger passivation film in addition to normal electrolytic polishing (EP) for customers who demand higher surface quality.
|
Pre-Electrolytic polishing solution |
Electrolytic polishing solution |
G-EP Processing |
P-EP processing |
|---|---|---|---|
|
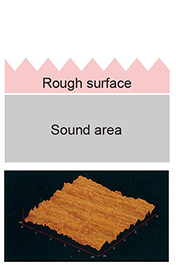
The heterogeneous oxidation state and granular particles are easy to get rid of after the surface is affected. |
Eliminate uniform oxidation state by Electropolishing. The surface becomes a flat and smooth metal surface. |
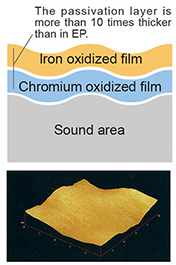
Burn the object in an oxidizing atmosphere. The passivation layer of the chromium oxide film grows and increases in thickness. Furthermore, the passivation layer of the iron oxide film covers the upper layer. |
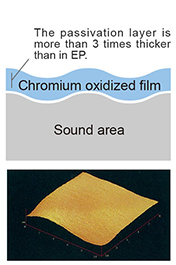
Remove the iron oxide film from the surface with a chemical solution. The surface layer is a passive layer of a chromium oxide film that is three times as thick as a general EP.. |
 |
 |
 |
 |
|
The surface of the metal is strongly affected by physical processing such as deterioration of the crystal structure and adhesion of dirt. |
A chromium-enriched layer with excellent corrosion resistance is formed. Its thickness is about 1 to 3 nanometers. |
This surface of metal suppresses the elution of metal ions in alcohol liquid or ozone water having a large amount of residual oxygen. |
This surface of metal suppresses the elution of metal ions in ultrapure water or organic amine solution with strong corrosiveness. |
【Fig. 6-1】 Comparison of general electrolytic polishing (EP) with GOLD EP and PLATINUM EP
* G-EP stands for GOLD EP, and P-EP stands for PLATINUM EP.
Features of GOLD EP/PLATINUM EP
-
Effective against elution of metal ions and TOC (total organic carbon) pollution
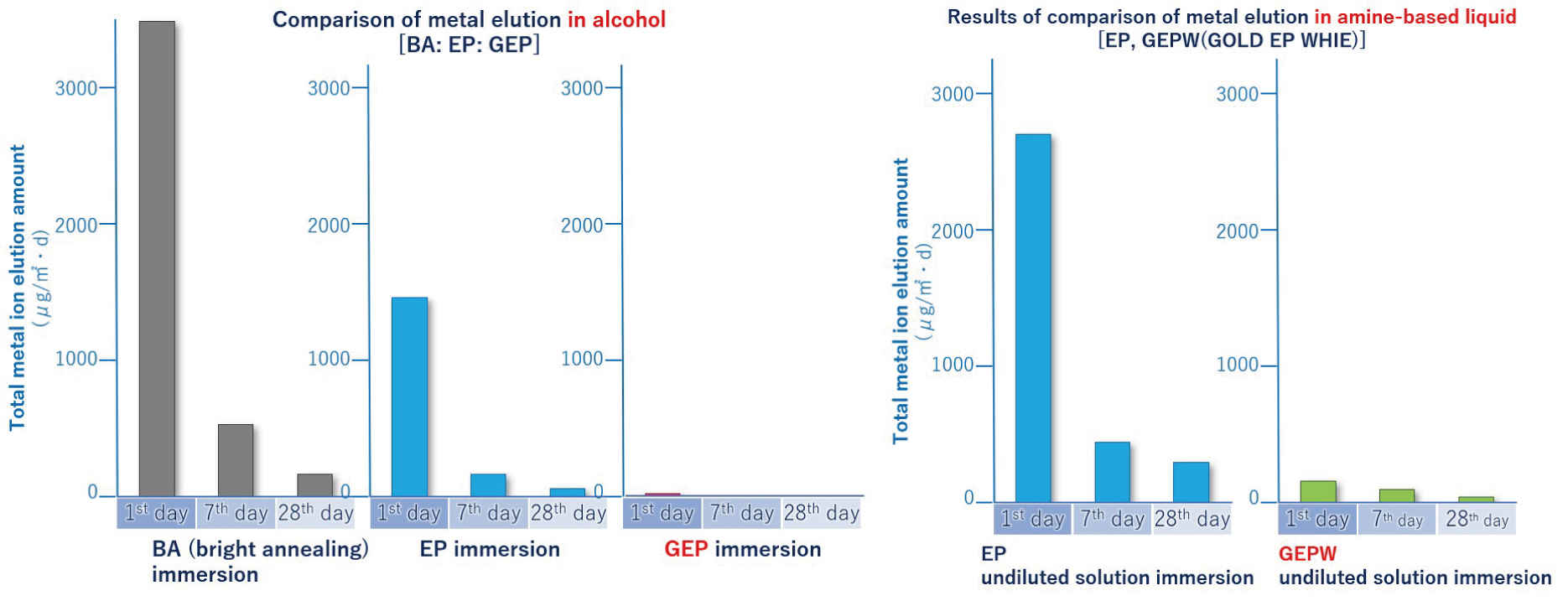
GOLD EP: Strong against elution with alcohol and ozone water with a lot of residual oxygen
PLATINUM EP: Strong against elution with ultrapure water and organic amine chemicals -
Resulting in a highly smooth surface which is resistant to particles like dust, contamination, etc.)
-
Excellent heat resistance and pressure resistance, and has a wide range of applications
-
Excellent workability such as bending, ferrule connection, and welding.
-
Mechanical strength is equivalent to stainless steel
-
Enhanced corrosion resistance by forming a thick passivation film (in the case of GOLD EP, the film thickness is about 10 times that of general EP)
【Figure 6-2】 Comparison between EP and GOLD EP / PLATINUM EP
* G-EP stands for GOLD EP, and P-EP stands for PLATINUM EP.
We will post a photo of a general ferrule-joint that has been electropolished by us.
(1) Electrolytic polishing of sanitary fittings / GOLD EP
The left side is a 90 degree elbow after a general EP (TEE 2.0S in the back), and the right side is a GOLD EP with an iron oxide film formed on the stainless steel surface by further heat treatment. There is no big difference visually except for the color, but GOLD EP has a strong passivation film that is about 10 times thicker than general EP.

【Picture 6-3】Appearance comparison of fittings with general electrolytic polishing (EP) and GOLD EP
(2)Samples of sanitary pipes after GOLD EP/PLATINUM EP
The golden pipe is GOLD EP and the white is PLATINUM EP. PLATINUM EP is the result after the iron oxide film of GOLD EP formed by heat treatment removed by chemical treatment. Under the removed iron oxide film, a passivation film of a thick chromium oxide film (about 3 times that of general EP) is formed, so elution to ultrapure water and organic amine chemicals is minimal.


【Picture 6-4】 Samples of cut pipes after GOLD EP, PLATINUM EP
(3) Rotor after GOLD EP
Not only piping through which products pass, but also machinery exposed to droplets such as seasonings and corrosive liquids, require corrosion resistance measures for important parts to ensure long-term operation.
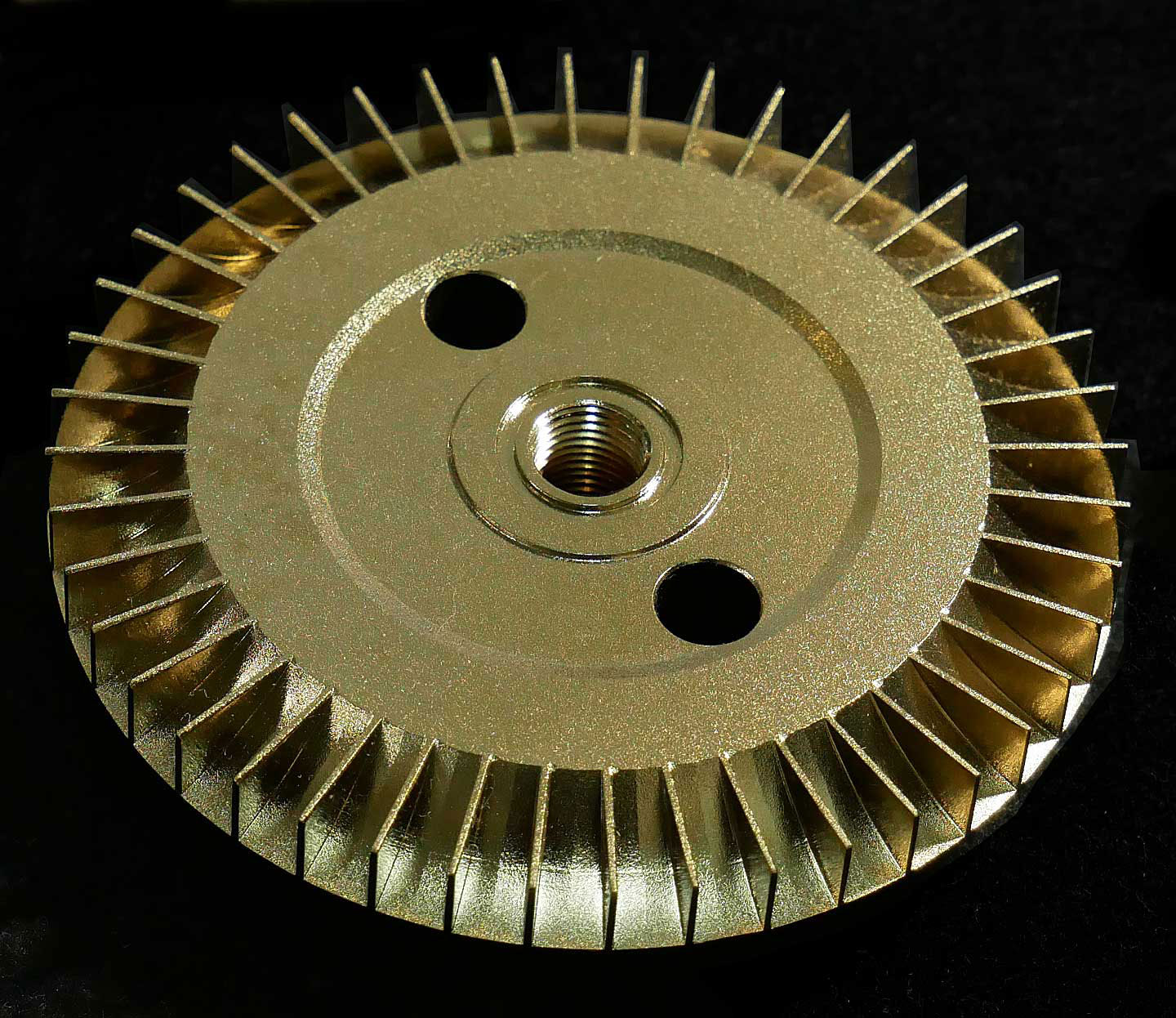
【Picture 6-5】Appearance comparison of Fittings with general electrolytic polishing (EP) and GOLD EP
7.Electrolytic polishing for materials other than stainless steel
VINA ASTEC CO., LTD., – our local subsidiary in Vietnam, has begun doing electrolytic polishing for active metals such as titanium (Ti) and aluminum (Al) in addition to stainless steel (Fe).
(1) EP for aluminum material
Below is an electrolytic polishing sample (round bar) of aluminum. The left side is the photo before EP and the right side is the photo after EP. The finish is different between the cross section and the side surface of the round bar, but when rolling metal into a round bar, the crystal structure has a directionality, so the cross section has a satin finish. The satinization also appears in the EP of stainless steel round bars.
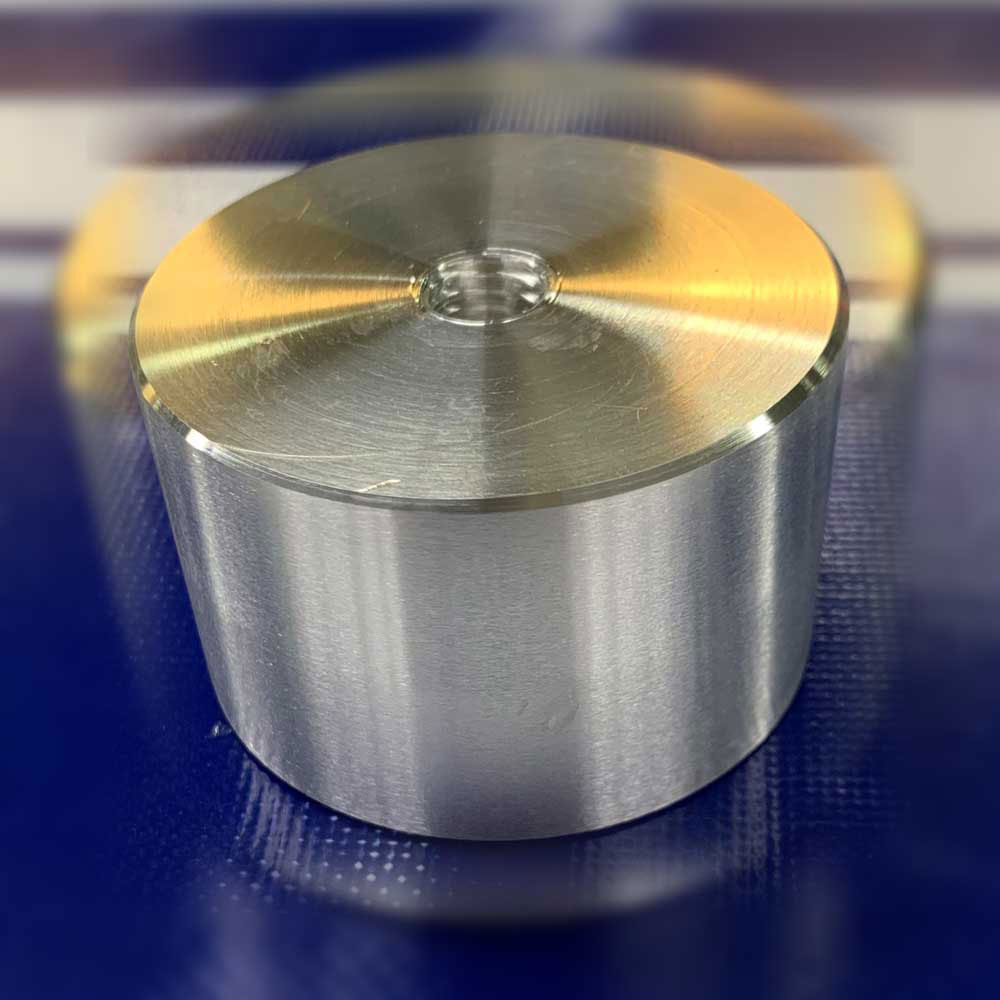
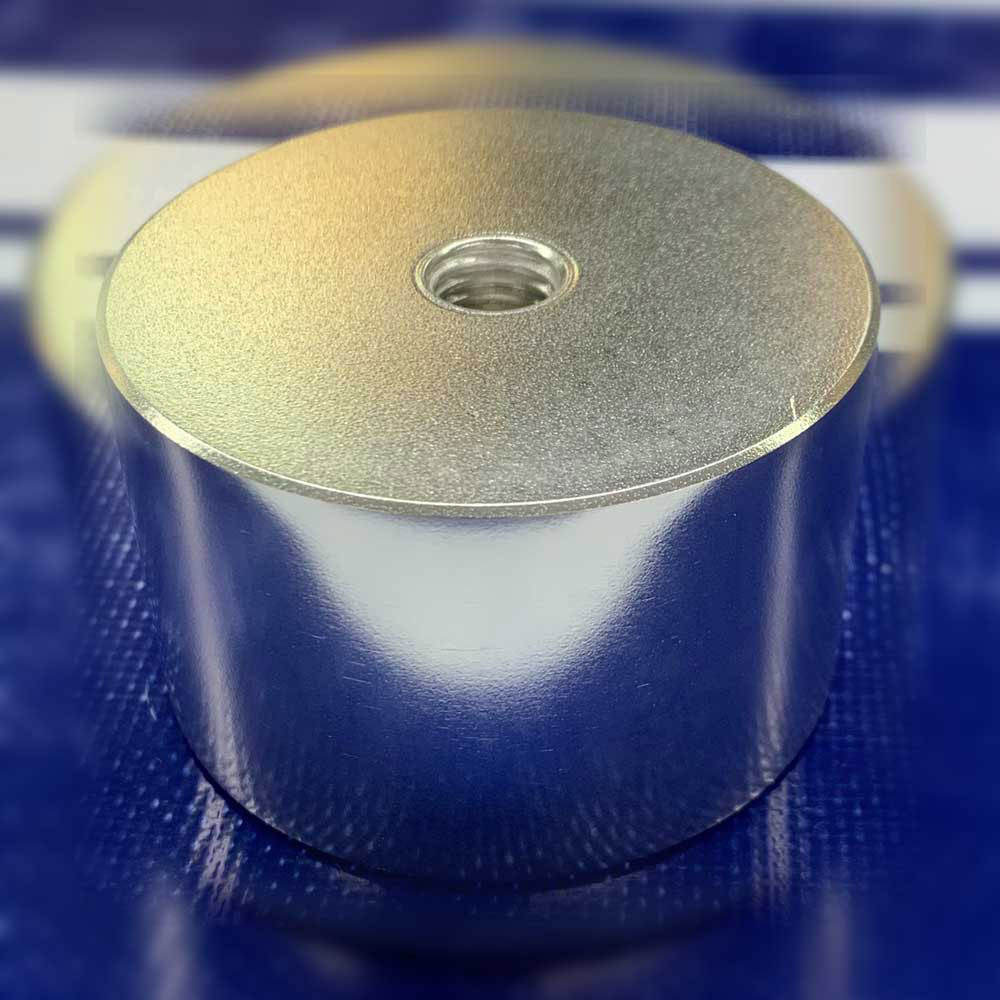
【Picture 7-1】Comparison of appearance before and after e electrolytic polishing of aluminum round bar
(2) EP for titanium material
This is an electrolytic polishing sample for titanium part.


【Picture 7-2】Comparison of appearance before and after electrolytic polishing of titanium part
(3) Coloring for titanium material
Color development by the titanium anodized film is caused by the interference between the metal surface reflected light and the oxide film surface reflected light. By controlling the thickness of the oxide film (transparent) formed, it is possible to attain the desired color.


【Picture 7-3】Coloring by forming an oxide film on titanium screws
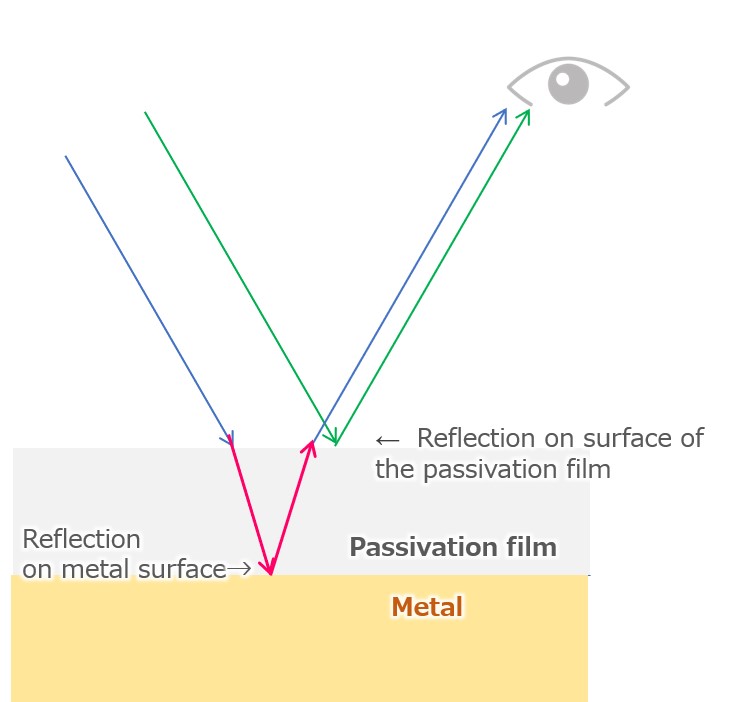
【Figure 7-4】interference of reflected light(phase shift)
The light reflected by the passivation film covering titanium object and the light reflected by the metal surface differ by the distance that passes through the passivation even with the same light source (red part in Fig. 7-4 on the left), and therefore the phase shift. As a result, a phenomenon called “light interference” occurs in which light of a certain wavelength (color) is weakened or strengthened.
Unlike painting and dyeing, titanium coloring is done using this interference of light, so even if you scrape the metal, the paint of the color you can see does not come out. By making the thickness of the passivation film uniform throughout the object, the entire metal looks the same color, and by controlling the thickness of the passivation film, the visible color differs.
Active metal
Metals with low ionization energy (weak ability to hold electrons to their own atoms) are easily ionized and are called “active metals”. Iron, aluminum, titanium, etc. are active metals that easily dissolve by reacting with water or acid. In addition, titanium and aluminum are metals with much higher activity than iron and can be said to be “prone to corrosion”. However, since the eluted ions immediately form a solid oxide film (passivation film), it is practically used as a very stable metal. Note that these highly active metals also generate gas actively during electrolytic polishing process.
8.Necessity to consider requirements for processing steps before and after electrolytic polishing
In 1985 (Showa 60), the blockbuster Super Mario Bros. was released, and, when Nippon Telegraph and Telephone Public Corporation was privatized and became NTT, we commercialized electrolytic polishing. In addition, since 2015, our Vietnamese subsidiary VINA ASTEC CO., LTD. has been conducting electrolytic polishing of Japanese quality in Vietnam.
Shape and size of products to be polished
We also have abundant know-how in processing long pipes, fittings, and custom-made products of various shapes, such as operations for performing uniform and high-quality electrolytic polishing, and electrodes and jigs.
【Fig. 8】 Actual electrolytic polishing (EP) (published on YouTube))
Surface treatment quality including pre- and post-processed
At our factories, we can handle everything from machining to surface treatment and installation work, and we are always aware that our products will operate in our customers’ manufacturing facilities and storage facilities.
Therefore, we place importance on welding and buffing in the previous process, which affect the quality of electrolytic polishing. We also pay close attention to post-processes which enhance the passivation film strength, various cleaning and then packing process. As we have an integrated working process, Our electrolytic polishing is unwavering high quality.
Information such as numerical values and graphs used in this content is mainly quoted from our “electrolytic polishing Technology Data”.